Gudermann/Chubanov Lab "Melastatin-related TRP Channels"
Head: Dr. Vladimir Chubanov
Technical assistance
Joanna Zaißerer
Doctoral students
Miyuki Egawa: Cellular functions of TRPM channels
M.D. students
Anna Rössig (FoeFoLe fellow): TRPM channels as new drug targets
Student and scientific assistance
Anna Erbacher
Lisa Pleninger
Completed dissertations
Moritz Meißner (M.D.)
Annika Wisnowsky (Dr. rer. nat.)
Silvia Ferioli (Dr. rer. nat.)
Sebastian Schäfer (M.D., FoeFoLe fellow)
Benjamin Stadlbauer (M.D., FoeFoLe fellow),
Lorenz Mittermeier (Dr. rer. nat.)
Banu Akdogan (PhD): TRPM channels in respiratory system
Eva Schmidt (Dr. rer. nat): Biophysical assessment of TRPM channels
The group’s main interest is a newly discovered group of plasma membrane proteins entitled as TRPM6 and TRPM7. These remarkable proteins comprise two distinct domains: an ion channel segment and a protein kinase domain. The channel segments of TRPM6 and TRPM7 have a high homology to the melastatin-related TRP (TRPM) channels, while the kinase domains resemble the α-type of serine/threonine protein kinases.
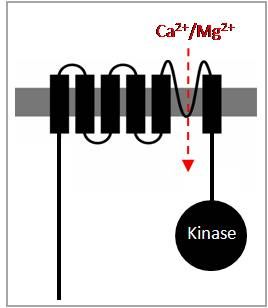
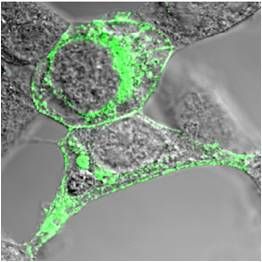
| Fig. 1: TRPM6 and TRPM7 are kinase-linked channels | Fig. 2: Immunolocalization of recombinant TRPM7 on the cell surface of HEK293 cells |
First functional information about TRPM genes has been obtained only a few years ago. Nevertheless, these studies have already resulted in several remarkable discoveries. For instance, TRPM8 channel was found as a cellular sensor for cold temperature and as a receptor for menthol. Menthol is known as one of the most ancient local anaesthetic drugs and food supplements. More recently, it was found that TRPM5 channel is essential for chemosensory function of taste receptor cells, olfactory neurones and recently identified sensory cells located in the lung and gastrointestinal tract.
The channel pores formed by TRPM6/M7 allow the passage of Ca2+, Mg2+ and other divalent cations into a cell. TRPM6/M7-like currents have been detected in all mammalian cells examined so far, indicating that this channel type plays an indispensable cellular role. It has been suggested that TRPM6/M7-mediated cation entries regulate Mg2+ homeostasis, cell spreading, proliferation, mechanosensitivity, exocytosis and other cellular processes.
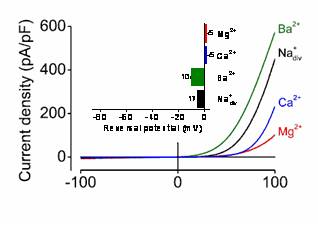
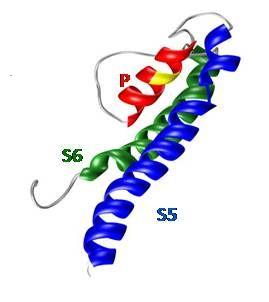
| Fig. 3: Patch-clamp measurements of the whole-cell currents mediated by TRPM7 | Fig. 4: 3D model of the pore-forming segment of TRPM6 |
Physiological implications of α-kinases are less understood. The best-studied α-kinase is elongation factor-2 kinase, which regulates a rate of the protein synthesis in response to nutrient deprivations and ER stress. Recently, annexin A1 and several myosin II isoforms have been identified as putative physiological substrates of the TRPM6/M7 kinases.
To summarize, initial studies revealed that TRPM6 and TRPM7 are multifunctional proteins required for many important cellular processes. However, unlike to other TRP channels, we still know very little about these remarkable proteins. Accordingly, my research group carries out the following research projects.
Current Projects
- We investigate a contribution of different domains and structural motives to functional properties of TRPM6/M7. To address this goal, we employ a broad spectrum of molecular biology, biochemical and biophysical techniques. For example, we use the site-directed mutagenesis in order to change critical amino-acid residues of TRPM6 and TRPM7 and, then, assess an impact of these changes on functional properties of the channels using the patch-clamp techniques and single-cell Ca2+ imaging. To monitor assembly and trafficking of TRPM6/M7 channel complexes we employ fluorescence resonance energy transfer (FRET) approach and confocal laser scanning microscopy. Overall, these studies will help us resolve which functional properties of the kinase-linked channels are central for their cellular role.
- Expression patterns of a given gene represent unbiased and indispensable information about its physiological role. To this end we generated a set of TRPM6 and TRPM7-specific antibodies. Currently we use these reagents in order to detect native proteins in mouse tissues and, in some instances, to establish their localization in distinct subcellular compartments.
- Experimental drugs acting as specific openers and blockers of TRPM6/M7 are not yet available. Therefore we perform a high-throughput screening (HTS) of chemical compounds targeting TRPM6 and TRPM7.
- We study TRPM6-deficient mouse in order to assess a role of TRPM6 in vivo. These investigations include comprehensive phenotypic and molecular analysis of TRPM6-/- mice and cells derived thereof.
Publications (German)

